 The National Human Rights Commission (NHRC) has sought action taken report from Centre over manufacturing and marketing of medicines across India with manufacturing date of April 2016.
The National Human Rights Commission (NHRC) has sought action taken report from Centre over manufacturing and marketing of medicines across India with manufacturing date of April 2016.
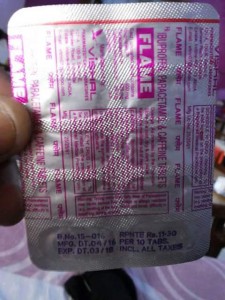
After a pack of a drug called FLAME (Ibuprofen, paracetamol and caffeine combination) has been found in different parts of the country with manufacturing date of April 2016, an Odisha rights activist Akhand from Civil Society Forum on Human Rights (CSFHR) had filled the petition before the apex rights panel demanding inquiring into the incident.
The Commission has issued notice the secretary of Ministry of Health and Family Welfare, Government of India to file an action taken report within four weeks.
The Medicines were manufactured by Vishal Pharmaceuticals Laboratories of Indore in Madhya Pradesh and supplied to the different medicines wholesalers and retailers throughout the country.
Incorrect manufacturing and expiry dates on medicines can have lethal consequences for patients. Expired medicines lose part or all of their potency, which means these medicines, is no longer as effective as they should be, the Petition Said.
“A patient who expects a medicine to cure him may not get any better if the medicine is an expired one, thus threatening his well-being and life”, said Akhand.
The administration has also remained silent on the matter. Health department officials are not taking necessary steps to check sale of fake drugs in the market. Even these departments are not carrying regular inspection of medicine stores to find out whether fake drugs are being sold by them or not, alleged Akhand.
Akhand had prayed for an inquiry and action against the pharmaceuticals company. Taking cognizance in the matter, the NHRC has sough report form Health Ministry. (IANS)
